Abdominal Aortic Aneurysm (Aaa)
- Home
- Abdominal Aortic Aneurysm (Aaa)
Abdominal Aortic Aneurysm (Aaa)
Introduction
The “Aorta” is the main artery that originates from the heart and supplies blood to entire body through its branches. In the part in the chest above the diaphragm it is called the “Thoracic Aorta”. It is shaped like an inverted hockey stick and the part going up is called “Ascending Aorta”, the curved part that gives off branches to both the arms, neck and head is called “Arch of Aorta” and the part coming down towards the legs is called “Descending Thoracic Aorta”.
The “Abdominal Aorta” is the part of the aorta that is located in the abdomen below the level of the diaphragm and extends down to its bifurcation into the two iliac arteries that supply both the legs. The part above the Renal Arteries (arteries to the kidneys) is called “Supra-renal Abdominal Aorta”, part below is called “Infra-renal Aorta” and the part close to the renals is called “Juxta-renal Aorta”.

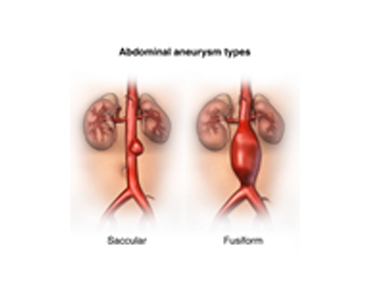
The wall of this artery is quite strong unless it gets weakened due to disease or infection or inherent weakness when it starts ballooning out and enlarging when it is called ectatic if it is <1.5x the normal size or aneurysm when it is >1.5x in size. This ballooning can happen either be uniform (fusiform aneurysm) or in part as a sac (sacular).
Like any balloon that gets inflated, as the size of the balloon gets bigger the wall becomes thinner and at some point it bursts or ruptures leading to bleeding and death.
The inner lumen of the aneurysm shows deposition of blood that clots (thrombus) and forms layers (Laminated Thrombus) that is adherent to the wall. Occasionally it may break away in parts, flow downstream and result in damage to the tissues (Thrash foot).

This is pathology is true for any artery in the body and this part of the information will focus on the Infra-renal part of the aorta (AAA). The aneurysmal part may also extend at times to involve the iliac arteries on one or both sides.
Once the aorta becomes aneurysmal, it usually increases @ 0.5cm per year in 60% of individuals, faster in 20% and slower in 20%. Hence periodic monitoring is always required.
The risk of rupture increases significantly once it gets >5cm in diameter (50% within 5 years) and the risk increases exponentially as it gets bigger. Saccular aneursyms are at higher risk of rupture than fusiform aneurysms.
Causes of Aortic Aneurysm
Risk factors include genetic factors or family history of aneurysm disease, age > 65 years, male sex, smoker, high cholesterol, high BP, diabetes, obesity, connective tissue disorder that causes weakening of wall due to faulty collagen, infection following Salmonella, Syphilis, myocbacteria, fungal (mycotic).
Symptoms
- 75% of cases are asymptomatic and are picked up incidentally on either abdominal ultrasound or CT scan performed for some under condition.
- Pain in the abdomen near the back is present when the increase is rapid.
- It can present as rupture with severe pain and may be associated with drop in blood pressure and collapse where there is a risk to life.
- Clots or thrombus present along the wall of the artery can also break away down stream and damage the skin or muscles of the legs or feet. (Thrash Foot).
Diagnosis
Large aneurysm can be seen as a bulge that pulsates when the patient is lying down and the abdominal wall is relaxed. On palpation, a pulsatile mass is felt and attempts are made to see if it is tender and if one can reach above the level of the aneurysm.
The diagnosis of the aneurysm and monitoring of its size is done commonly using an Abdominal Ultrasound.
CT scan with angiography is done when one considers going ahead with treatment and to plan the best line of treatment.
Assessment also involves identifying the risk factors and establishing the cardiac and respiratory risk for General Anesthesia and Surgery.
Treatment
Treatment starts with modification and control of risk factors. Medicines are given to lower the blood pressure to lower the risk of rupture.
If the aneurysm is <5cm in diameter, the patient is monitored every 6-12 months with serial Abdominal Ultrasound until it gets >5cm or the rate of increase is > 0.5cm per year.
If the aneurysm is larger >5cm then intervention is planned if the patient is fit for surgery. Open surgery is preferred for young, fit patients and if the aneurysm is very close to the renal arteries (<1.5cm) or extremely angulated or tortous. Endovascular Repair (EVAR) is preferred in older, less fit patients if the aneurysm morphology is suitable for a stent graft.
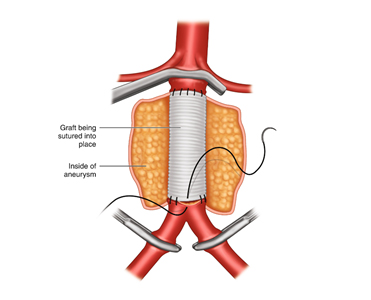 Open surgery is performed in the operation theatre and the abdomen is opened and the artery above (proximal) and below (distal) the aneurysm is dissected and controlled. After thinning the blood, vascular clamps are applied distal first (to prevent thrash foot) and then proximal to stop blood flowing into the aneurysm. The aneurysm is then opened and all the clot is removed. Any small branches that back bleed are sutured shut. An appropriately sized graft (dacron or PTFE) is then sutured to both the ends of the artery and blood flow restarted. If only the aorta is involved a straight tube is used and if the iliac arteries are also involved a bifurcated or trouser graft is used. The wall of the aneurysm is closed around the graft to prevent it from coming in contact with the bowel wall. The abdomen is closed and patient is either woken up or kept on ventilator till he becomes stable and is shifted to the ICU for observation.
Open surgery is performed in the operation theatre and the abdomen is opened and the artery above (proximal) and below (distal) the aneurysm is dissected and controlled. After thinning the blood, vascular clamps are applied distal first (to prevent thrash foot) and then proximal to stop blood flowing into the aneurysm. The aneurysm is then opened and all the clot is removed. Any small branches that back bleed are sutured shut. An appropriately sized graft (dacron or PTFE) is then sutured to both the ends of the artery and blood flow restarted. If only the aorta is involved a straight tube is used and if the iliac arteries are also involved a bifurcated or trouser graft is used. The wall of the aneurysm is closed around the graft to prevent it from coming in contact with the bowel wall. The abdomen is closed and patient is either woken up or kept on ventilator till he becomes stable and is shifted to the ICU for observation.
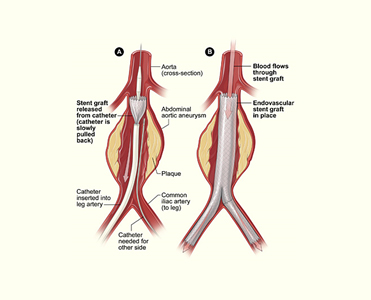 EVAR is usually performed in the Cathlab or a Hybrid OT and the abdomen is not opened up. Instead, a small cut is made in one or both groins and the groin artery (femoral) is dissected and opened up after thinning the blood and clamping. The stent graft is then deployed over a wire under Xray guidance using an Image Intensifier. The upper end of the graft is deployed just below the level of the renal arteries. The lower end is then deployed in the healthy iliac arteries below the level of the aneurysm. Angiogram is performed to confirm there is no filling up of the aneurysm and the femoral artery and wound is closed. Patient is usually woken up and kept in the ICU for a day or two.
EVAR is usually performed in the Cathlab or a Hybrid OT and the abdomen is not opened up. Instead, a small cut is made in one or both groins and the groin artery (femoral) is dissected and opened up after thinning the blood and clamping. The stent graft is then deployed over a wire under Xray guidance using an Image Intensifier. The upper end of the graft is deployed just below the level of the renal arteries. The lower end is then deployed in the healthy iliac arteries below the level of the aneurysm. Angiogram is performed to confirm there is no filling up of the aneurysm and the femoral artery and wound is closed. Patient is usually woken up and kept in the ICU for a day or two.
Patient needs to be monitored at regular intervals in the future with Ultrasound or CT angiography to ensure that there is no dislodgement or migration of the stent graft and there is no leaking of blood into the aneurysm sac.


